1.23 -1.24 Grouping together
1.23 Family resemblances
Students should:
1.23 understand why elements in the same group of the Periodic Table have similar chemical properties
1.24 understand why the noble gases (Group 0) do not readily react
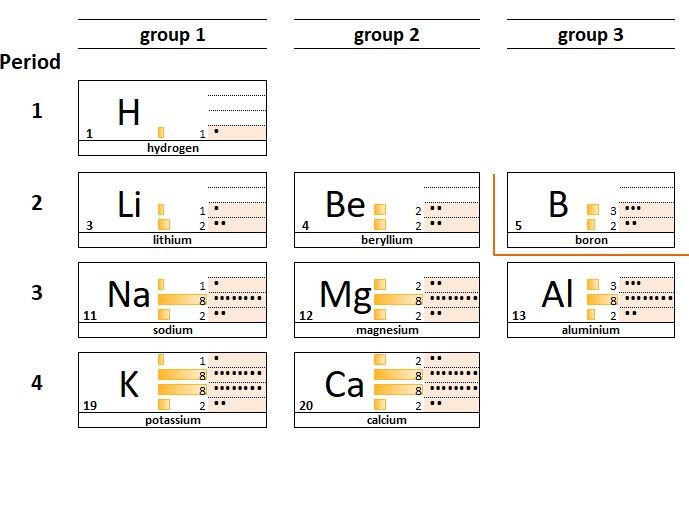
Looking at the electron arrangements of the atoms in groups 1, 2 and 3 you can see that the atoms of group 1 elements have 1 electron in their outermost shell, group 2 elements all have 2 electrons and group 3 elements 3. This carries on in groups 4,5,6,7.
This similarity of electron configuration within a given group of elements explains why the elements in a group behave in similar ways; they have similar physical and chemical properties.
For example: lithium Li , sodium Na, and potassium K :
- all react violently with water.
- are all soft low density metals
- combine with chlorine atoms in a 1:1 ratio
- form oxides which dissolve to give an alkaline solution
1.24. Activity 4. A Noble family
The chemical behaviour of a element is partly determined by the number of electrons in the outer shell.
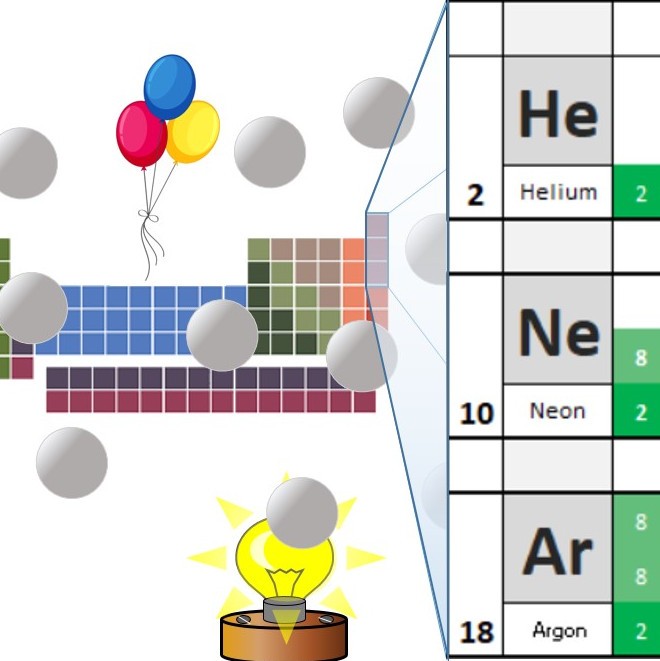
In the noble gases or group 0 the outermost shell of the atoms contains 8 electrons (2 in case of Helium) which is a stable state configuration.
Hence, the noble gases are chemically unreactive or inert.
noble, inert, 8 or 0?
In the shortened periodic table, the group number is the same as the number of outer shell electrons in the atoms of that group. This is not true for the noble gases. Helium only has 2 electrons in its outer shell, while the others all have 8 . Because of this, the group number was changed to 0 .
Originally the group was known as the inert gases as it was thought that they would not react with anything. However in 1962 a chemist called Bartlett made a compound from Xenon. The preferred name for the group is now the noble gases.
-
Why is argon used to fill light bulbs ? Open or Close
 Argon gas is used to surround the filament in incandescent light bulbs. Argon is inert and therefore does not react with the white hot filament. If air was used the filament would quickly react with the oxygen in it and the lamp would blow.
Argon gas is used to surround the filament in incandescent light bulbs. Argon is inert and therefore does not react with the white hot filament. If air was used the filament would quickly react with the oxygen in it and the lamp would blow. -
Why is helium rather than hydrogen preferred for use in airships? Open or Close
Helium and hydrogen are both less dense than air and so can both be used to provide the uplift for a airship. However, hydrogen is very flammable and was the cause of the Hindenburg disaster in 1937.
Helium is inert and is therefore quite safe to use in gas balloons and airships.
-
What does the word inert mean ? Open or Close
Inert is used to refer to a substance that does not react with others. The noble gases are all fairly inert due to the full outer shell of electrons.
Nitrogen gas is quite inert due to its triple bond. The precious metals such as gold, platinum, and silver can also be describes as inert. The noble gases are thought to have been named after these noble metals.
-
Why is the preferred name for this group the "noble gases" Open or Close
For a long time, many people believed the noble gases to be completely nonreactive and unable to form chemical compounds. Although these elements don't form compounds readily, examples of molecules containing xenon, krypton, and radon have been found. At high pressure, even helium, neon, and argon participate in chemical reactions. The name "noble" is preferable to inert since strictly speaking they are not completely inert.
-
Did you know? Open or Close
- The first noble gas to be discovered was argon.
- Argon glows violet in a gas discharge tube. It is the gas found in plasma balls.
- Argon gas is prepared by fractionating liquid air.
- The Earth's atmosphere contains 0.94% argon.
- Mars' atmosphere contains 1.6% Argon
- The thin atmosphere of the planet Mercury is about 70% argon.

When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.