1.46 Covalent bonding - "making molecules"
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds.
1.46 Activity 2. Crossing and dotting
Students should:
- 1.46 understand how to use dot-and-cross diagrams to represent covalent bonds in molecules.
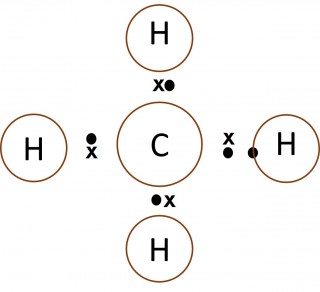
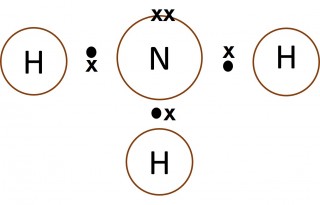
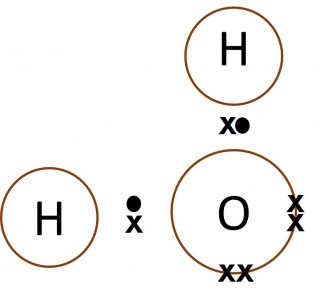
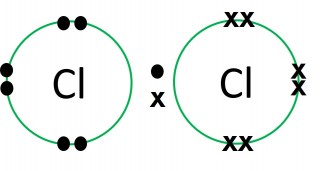
Dot and cross diagrams are a useful way of representing how atoms share electrons to form molecules.
Watch the video and notice that:
- Only the outer electrons need to be shown.
- The electrons on one of the atoms are shown as dots.
- The electrons on the other atom involved are shown as crosses.
- Normally a "dot" and a "cross" pair up to form an ordinary single covalent bond.
1.46 Activity. Four simple molecules
- Study the animation carefully.
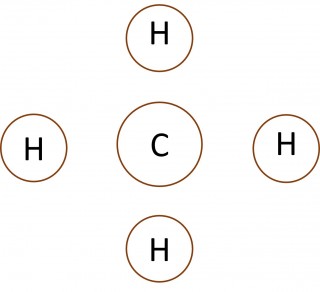
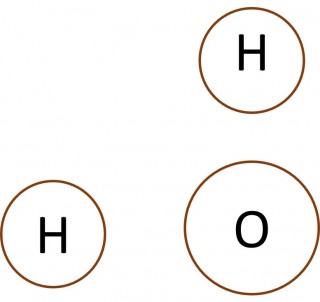
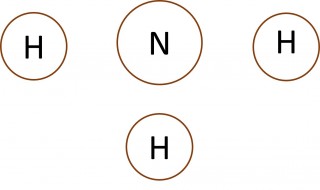
- What can you say about the number of electrons in the outer shell of the carbon atom in methane, the nitrogen atom in the ammonia molecule and the oxygen atom in the water molecule?
- Make a copy of each of the images below and add dots and crosses to them to show the complete electron configurations
1.46 Activity. Multiple Bonds
-
What is unusual about one of the bonds in carbon monoxide? Open or Close
The dot and cross diagram shows that a triple bond forms in the carbon monoxide molecule. It also shows that in one of the triple bonds, both electrons come from the oxygen atom.
This type of bond is still an ordinary covalent bond but is is known as a dative covalent bond. Such bonds are sometimes represented by an arrow.
1.46 Activity. Ethane, Ethene and Chloromethane
Study the dot and cross diagrams for each of the three molecules shown in the animation.
Notice that for ethane and ethene two different colours of cross have been used to distinguish the electrons associated with one of the carbon atoms from the electrons on the other. The same method is used to distinguish Chlorine's electrons from the carbon's electrons in the chloromethane.
Think about the formula, shape, structure and name of each of the molecules shown.
Predicting an unknown molecule
- Try to draw a 3d representation of chloroethene
- Try to draw a dot and cross diagram to show the electron arrangement in chloroethene
Chloroethene image
Chloroethene dot and cross
Activity. Complete the matching exercise below
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.