1.39 Ionic bonding - finding formulae
1.39 Activity. Finding formulae
Students should:
- 1.39 write formulae for compounds formed between the ions listed
Watch the video closely pausing where instructed. Use the information given to work out the correct formulae for the following compounds:
- lithium nitride
- potassium oxide
- zinc oxide
- silver chloride
- Li3N
- K2O
- ZnO
- AgCl
1.3.9 A neat trick
A simple way to work out the formula of an ionic compound.
You can use this method to balance the charges and therefore work out the formula of any combination of positive and negative ions.
Note :
If the charge is 1- or 1+ we do not put the number 1 in the formula. For example : for sodium chloride we write NaCl rather than Na1Cl1
If the charges are 2+ and 2- ( as in magnesium and oxygen) , the crossover method would give us Mg2O2 but we simplify this and write it as MgO.
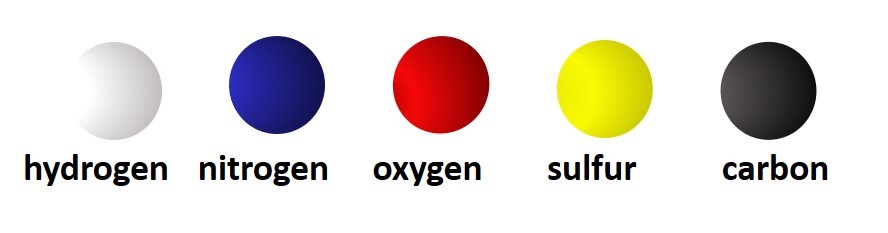
So far we have considered only monatomic ions. These are ions formed when single atoms gain or lose electrons.
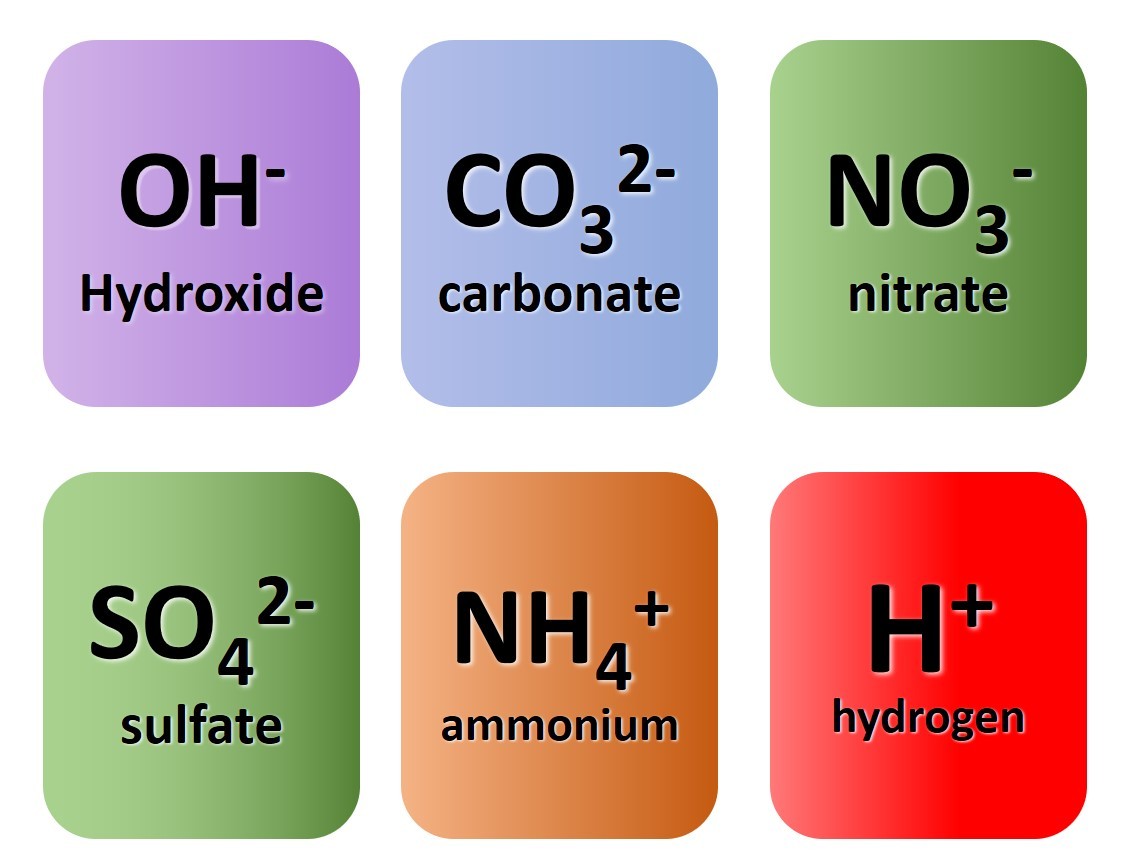
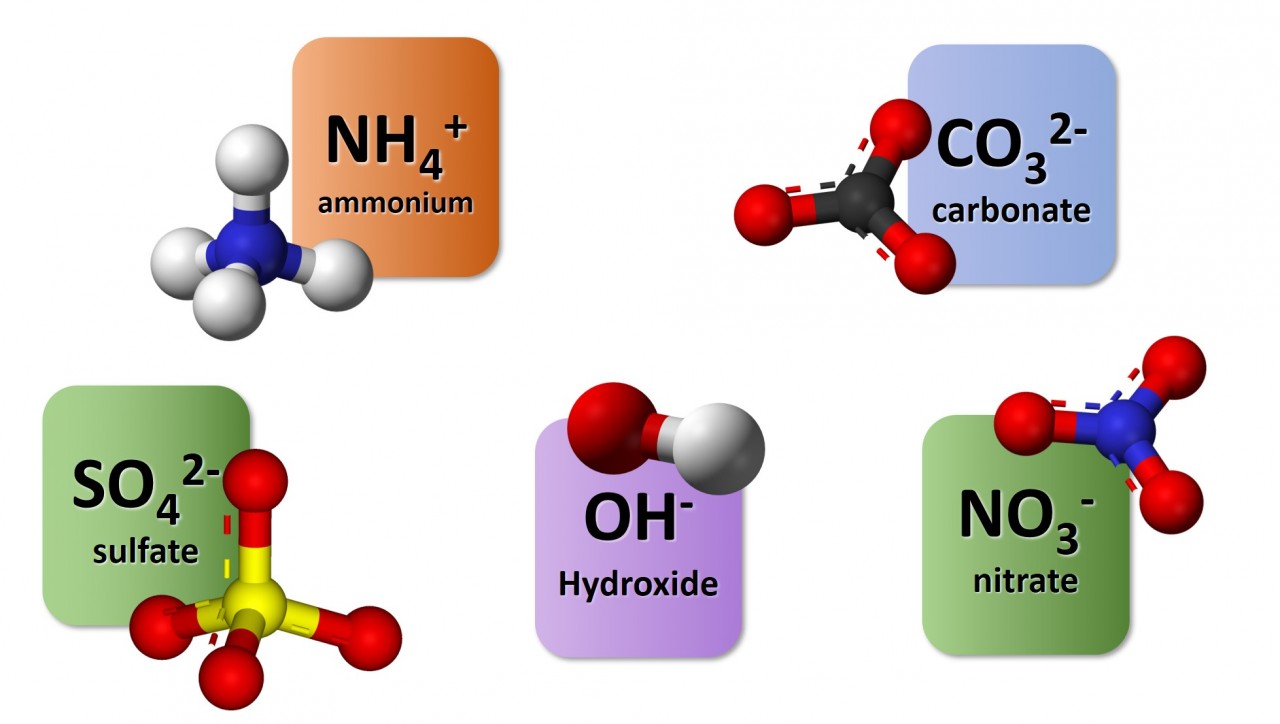
1.3.9 Activity. Polyatomic ions
1.3.9 Activity 4. Polyatomic formulae
When writing formula involving polyatomic ions you need to be careful to use round brackets appropriately. The video here explains why.
Watch the video carefully and use it to note down the formulae of the following compounds :
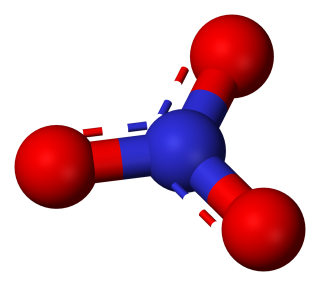
- sodium nitrate
- magnesium hydroxide
- potassium sulfate
- copper (II) chloride
- copper (I) oxide
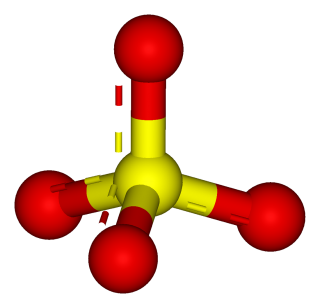
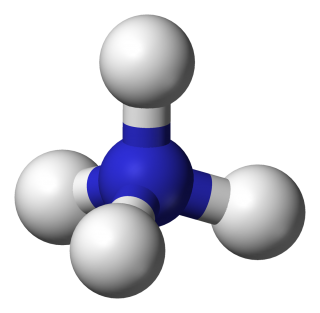
- ammonium sulfate
- aluminium carbonate
** Check your answers by rolling your cursor over the relevant name.
Activity 3. Complete the learning and matching exercises below to make sure you know all the ions and charges
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.