1.20 - 1.22 periodic patterns
1.20 - 1.22 Activity 2. Periodic variations
Students should:
1.20 understand how to use electrical conductivity and the acid-base character of oxides to classify elements as metals or non-metals
1.21 identify an element as a metal or a non-metal according to its position in the Periodic Table
1.22 understand how the electronic configuration of a main group element is related to its position in the Periodic Table
Good conductors?
Study the presentation to investigate and answer the following questions:
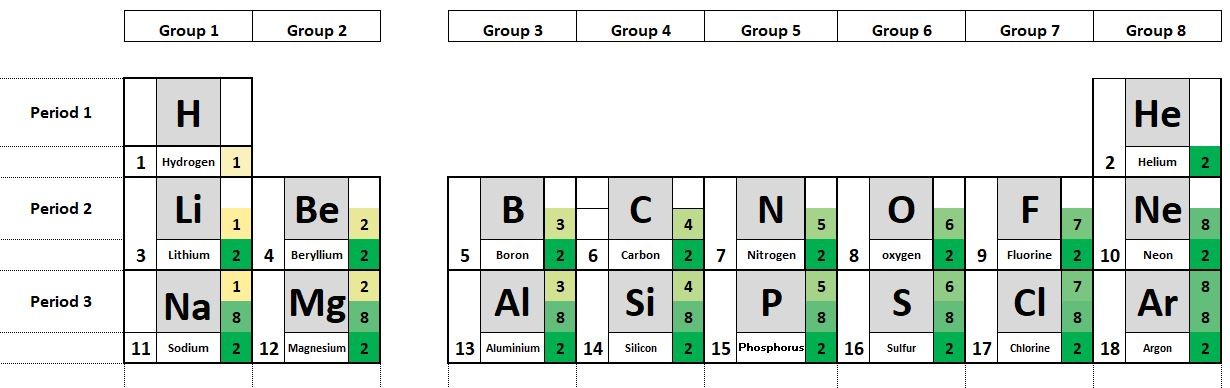
- What can you say about the connection between the number of electrons in the outer shell of the atoms and the group number shown?
- What can you say about the number of occupied electron shells and the period number.
- What can you say about the electron configuration of the noble gases?
- Where on the periodic table are the metals found?
- What can you say about the electrical conductivity of the metals compared with that of the non- metals?
The period number tells us the number of occupied electron shells in a given atom.
The group number shows the number of electrons in the outermost shell of the atom.
The atoms of the elements in group 8 have full outer shells of electrons
The metals can be found to the left and bottom of the periodic table.
The metals all conduct an electric current. The semi metals are semiconductors. The non-metals do not conduct an electric current.
Acid or base?
This video animation shows how the acid/base properties of the oxides varies across the periodic table. The pattern is not perfect partly because many non-metals can form more than one oxide and also because their solubility varies. However a general trend can be seen.
Stop the video after 21 seconds and try to predict what the trend will be. Test your prediction by watching the rest of the video.
What general conclusion can you make about the acidity or alkalinity of the oxides of :
- Metals?
- Non-Metals?
- Semi-metals?
The oxides of metals are generally alkaline ( basic) . The oxides of non metals are generally acidic.
The oxides of the semi-metal elements (e.g Si, Ge) tend to be "neutral" and not very soluble in water. These oxides will however dissolve in both acids and in alkalis. Such oxides are known as amphoteric oxides.
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.