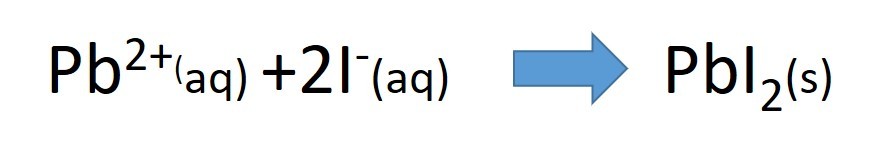2.34 - 2.43 Acids, alkalis and salt preparation
2.34 Defining salts
Common "table" salt ( sodium chloride) is found dissolved in large quantities in seawater. Sodium Chloride is just one example of the many compounds which can be called salts. Most salts are crystalline ionic compounds
A salt is defined as :
A compound resulting from a chemical reaction of an acid, in which the acid's hydrogen ions are replaced by other (positive) ions .
Assumed background knowledge

Ionic Bonding - transferring charge
2.34 Activity 1. The best solution?
Students should:
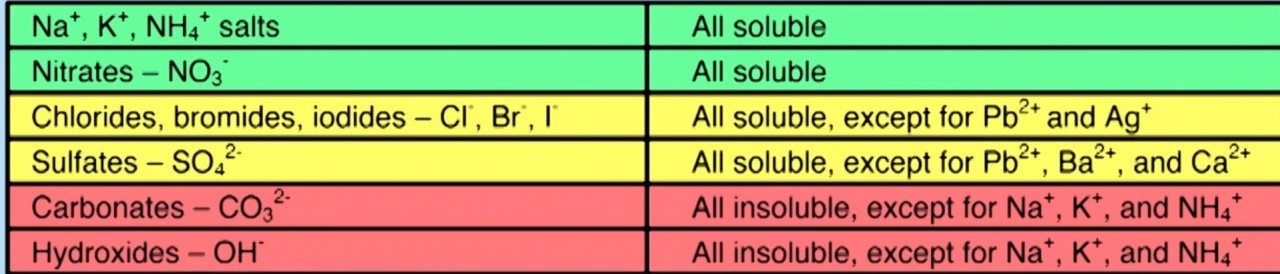
- 2.34 know the general rules for predicting the solubility of ionic compounds in water
Different Ions have different solubilities . When two soluble ions are combined to form a salt the salt will remain in solution. Thus a soluble salt is formed
When two insoluble ions mix to form a salt, the salt forms as a precipitate.
- What can you say about the solubility of barium sulfate?
- Why is this useful?
- How would you describe the solubility of calcium hydroxide solution?
- Barium sulfate is completely insoluble.
- This means it can be ingested without being absorbed by the body. Barium sulfate is view able in X-Rays. This means it can be used to find the intestinal tract in the body.
- Calcium hydroxide solution is mildly soluble.
Pretty precipitation:
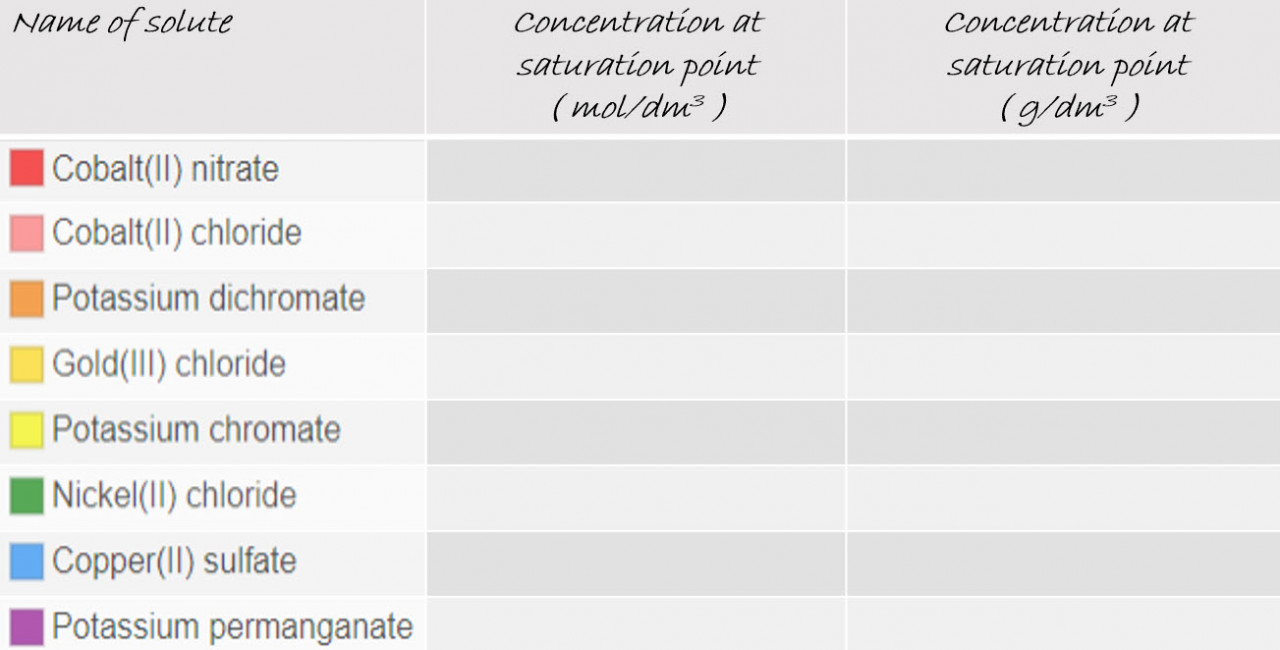
2.34 Activity 2. Saturation points
2.34 Activity 3. Predicting precipitates
Use the solubility table to predict what you would observe when you mix the following pairs of solutions:
- sodium sulfate solution + potassium chloride solution
- barium chloride solution + ammonium nitrate solution
- silver nitrate solution + copper chloride solution
- lead nitrate solution + potassium Iodide solution
- barium chloride solution + magnesium sulfate solution
2.35 Activity 4. Neutralisation
Students should:
- 2.35 understand acids and bases in terms of proton transfer
- 2.36 understand that an acid is a proton donor and a base is a proton acceptor
- 2.38 know that metal oxides, metal hydroxides and ammonia can act as bases, and that alkalis are bases that are soluble in water
- Bases and alkalis are both capable of neutralising acids. However they are not exactly the same thing. Explain.
- What is the name and formula of the anion ( negative ion) present in all the alkali In the examples considered in the video?
- How does the concentration of the anion mentioned in 2 affect the pH of the solution?
- When acid is added to alkali, which ions combine to make water?
- Why are neutralisation reactions useful?
Acids can be defined as substances which will donate protons ( hydrogen ions H+ )
Bases are defined as substances which will accept protons.
When an acid neutralizes a base, protons (H+ ions) are transferred from the acid to the base. This produces water and a salt.
2.37 - 2.39 Activity 5. Reacting Acids
Students should:
- 2.37 describe the reactions of hydrochloric acid, sulfuric acid and nitric acid with metals, bases and metal carbonates (excluding the reactions between nitric acid and metals) to form salts
Watch the animation carefully and write word equations giving the names the reactants and products for the four reaction types shown:
- acid and alkali react to form a soluble salt plus water
- acid and insoluble base react to form a soluble salt plus water
- acid plus metal react to form a soluble salt plus hydrogen gas
- acid plus carbonate react to form a soluble salt plus carbon dioxide plus water
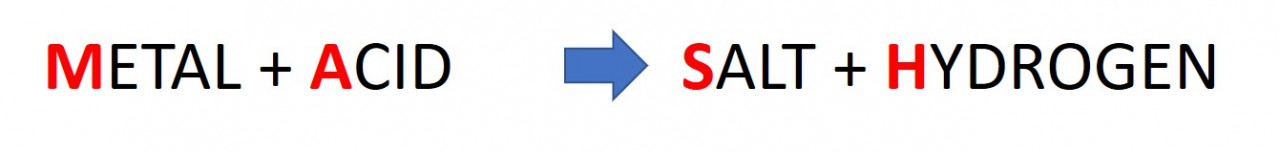
2.37. Metal plus Acid (MASH)
2.39, 2.40, 2.42 Activity 6. Making Soluble Salts
Students should:
- 2.39 describe an experiment to prepare a pure, dry sample of a soluble salt, starting from an insoluble reactant
- 2.40C describe an experiment to prepare a pure, dry sample of a soluble salt, starting from an acid and alkali
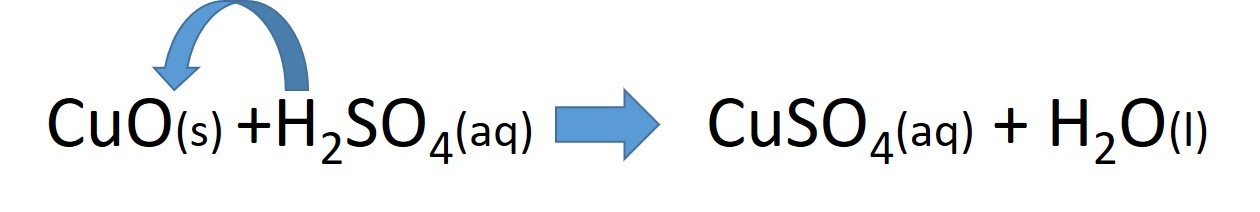
- 2.42 practical: prepare a sample of pure, dry hydrated copper(II) sulfate crystals starting from copper(II) oxide
Study the video and use it to construct a sequence of instructions to describe how to make a soluble salt :
- Add insoluble base to a volume of the acid (50cm3) in a beaker
- Titrate the solution with an indicator in to find the volume required for neutralisation
2.41, 2.43 Activity 7. Making Insoluble Salts
Students should:
- 2.41C describe an experiment to prepare a pure, dry sample of an insoluble salt, starting from two soluble reactants
- 2.43C practical: prepare a sample of pure, dry lead(II) sulfate
Watch the video then complete the missing word exercise below.
When an insoluble salt forms from two solutions by a ********** reaction the salt can be separated from the liquid by **********. This type of reaction is also known as a ********** reaction.
The solid salt remains as a ********** in the filter paper.
The residue should be cleaned by washing it with ********** water
It can then be dried by "patting" with dry filter paper or left out in the air to dry.
Missing words: distilled, filtration, precipitation, residue, double displacement
When an insoluble salt forms from two solutions by a precipitation reaction the salt can be separated from the liquid by filtration. This type of reaction is also known as a double displacement reaction.
The solid salt remains as a residue in the filter paper.
The residue should be cleaned by washing it with distilled water
It can then be dried by "patting" with dry filter paper or left out in the air to dry..