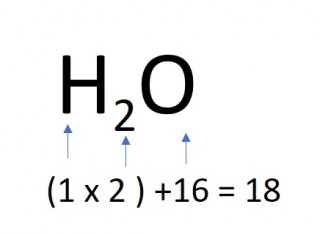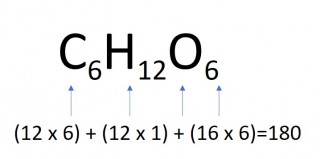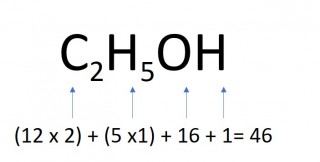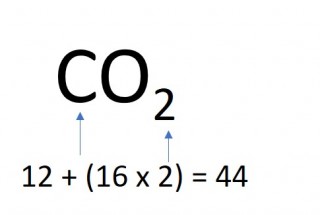1.28 Moles and mountains
1.28 Calculating moles
The following masses of elements all contain one mole of atoms:
- 12.0 g Carbon,
- 32.1 g Sulphur,
- 14 g Nitrogen,
- 24.3 g Magnesium.
So if you have 24 g carbon you have 24/12 = 2 moles carbon atoms.
Likewise if you have 1.4 g nitrogen you have 1.4/14 = 0.1 moles nitrogen atoms.
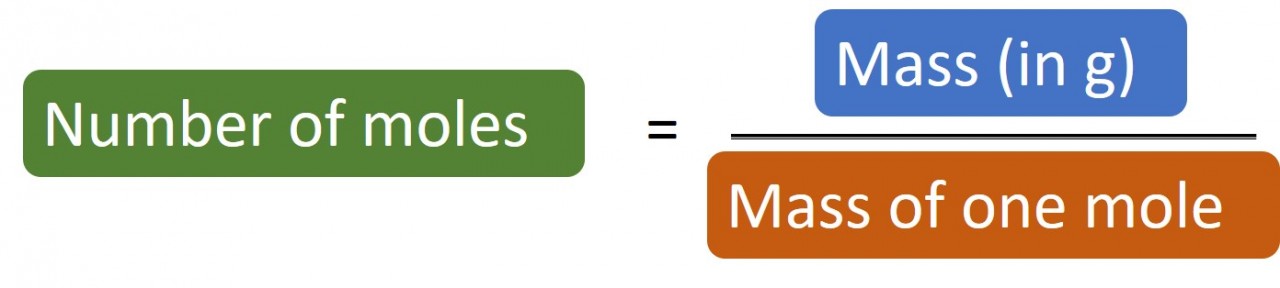
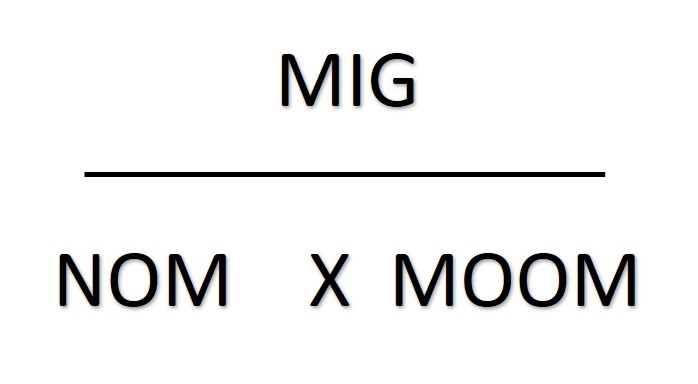
Rearranging things
The mass of one mole ("MOOM")is the relative atomic number for an element. If the substance is a compound you will need to work out its relative molecular mass or relative formula mass.
Activity 1. Moles from mass
Watch the short video to see how the number of moles can be calculated from the mass of the sample and the mass of one mole (Ar) . Use the same method to calculate the number of moles in the five samples given on the questions tab below. Use the answers tab to check your calculations:
- 355g of chlorine
- 0.69g of lithium
- 8.0g of oxygen
- 35g of copper
- one tonne of lead
There are:
- 10
- 0.1
- 0.5
- 0.55
- 4826.25
Activity 2. Mass from moles
Watch the short video to see how the mass of a sample can be calculated from the number of moles and the mass of one mole (Ar) . Use the same method to calculate the mass in the ten samples given on the questions tab below. Use the answers tab to check your calculations:
What is the mass of:
- 2.5 moles of oxygen atoms?
- 0.15 moles boron atoms
- 5 moles of aluminium atoms
- 0.4 moles of sulfur atoms
- 50 moles of oxygen atoms
- 1 mole of water molecules
- 0.5 moles of ethanol
- 4 moles of glucose
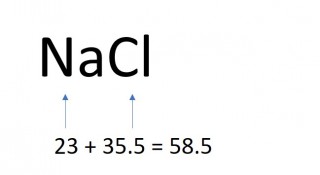
- 2 moles of sodium chloride
- 2 moles of carbon dioxide molecules
Enter your text here ...
Enter your text here ...
Enter your text here ...
Enter your text here ...
Enter your text here ...
- 2.5 x 16 = 40g
- 0.15 x 10.8 = 1.62g
- 5 x 27 = 135g
- 0.4 x 32.1 = 12.84g
- 50 x 16 = 800g
- 1 x 18 = 18g
- 0.5 x 46 = 23g
- 4 x 180 = 720g
- 2 x 58.5 = 117g
- 2 x 44 = 88g
Activity 3. Matching moles and masses
When you have successfully completed the calculations in activities 1 and 2 you should be able to test your skill using the quizlet below:
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.