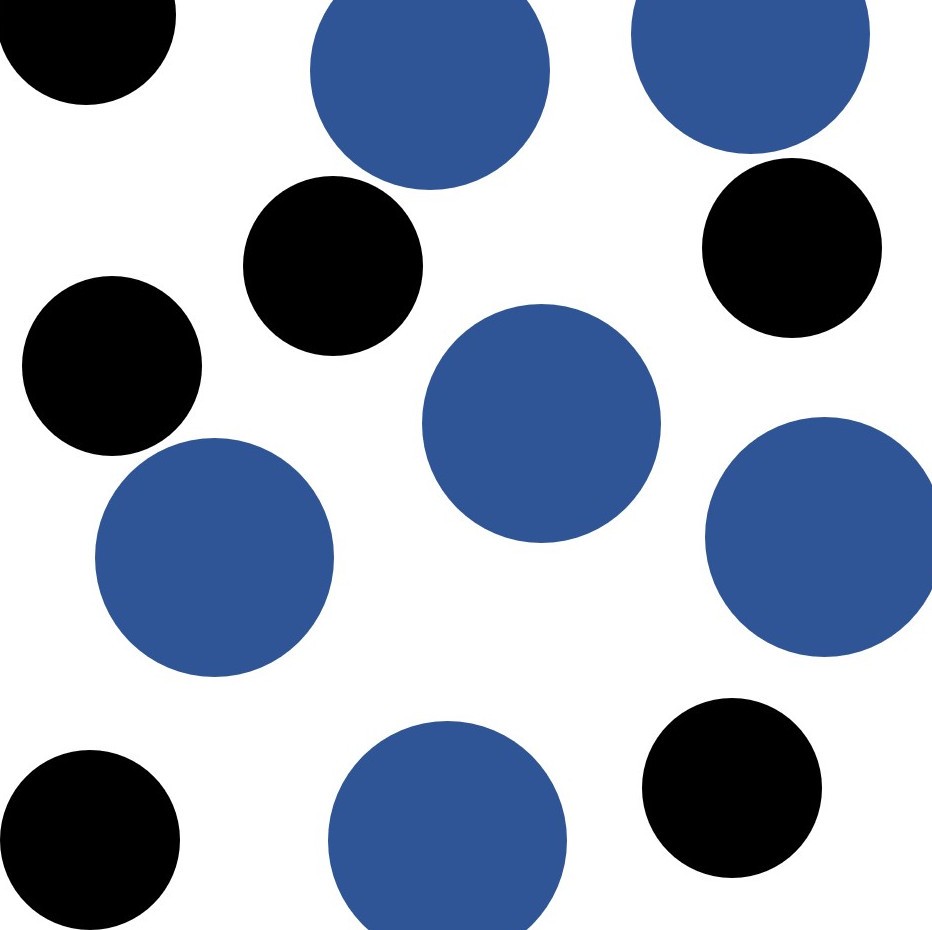
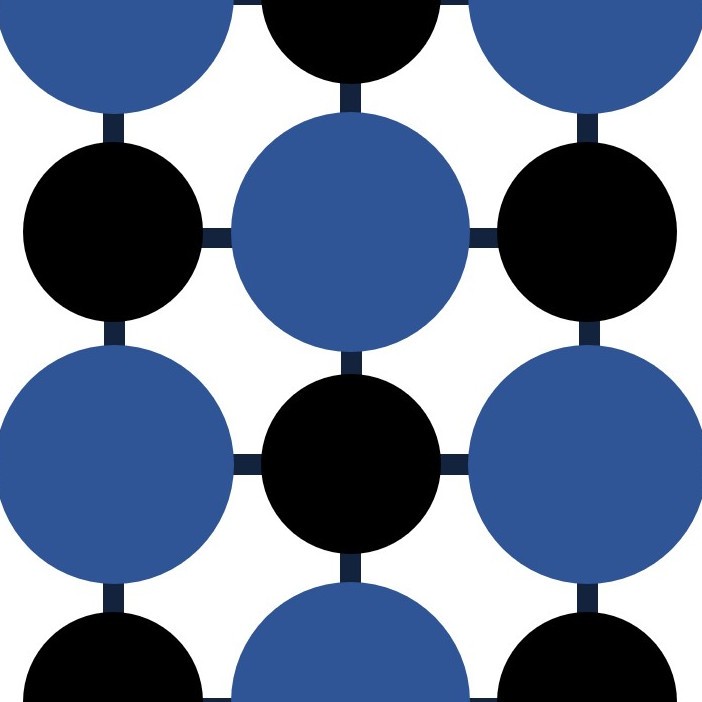
Compound or mixture?
Sun and air
Study the seaside image for a few seconds . Try to describe it:
You might say that the image shows : A calm sea, clear blue sky, bright sunshine and a perfectly smooth beach.
You could describe it in terms of the states of matter visible:
| SOLID | Sand |
| LIQUID | Sea |
| GAS | Air |
| GAS + PLASMA | Sun |
But as a chemist you might be asked to classify each of these four components as either mixtures, compounds or elements.
You might then go further and identify the atoms which are make up each of those components .
This section will help you to do that.
Mixed or bonded?
MIXTURE : A mixture is made up of two or more components which although mixed together can be separated by physical means. The components are not chemically combined.