Transition elements
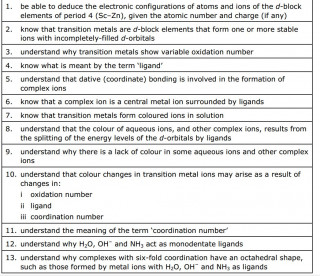
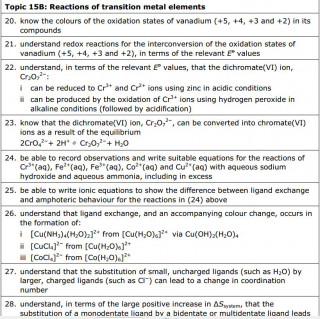
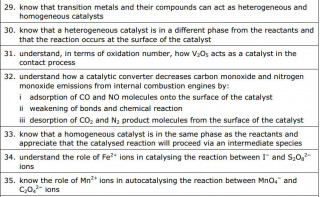
Electron configurations
The video here uses the relevant section of the Royal Society of Chemistry's periodic table website to show the electron configurations of the first row of the transition elements
Use the video to find out what is surprising about the electron configuration of
Chromium
Answer: The electron configuration of Chromium is given as [Ar] 4s1 3d5 - this is surprising as we might expect /predict [Ar] 4s2 3d4
Copper
Answer: The electron configuration of Copper is [Ar] 4s1 3d10 - this is surprising as we might expect /predict [Ar] 4s2 3d8
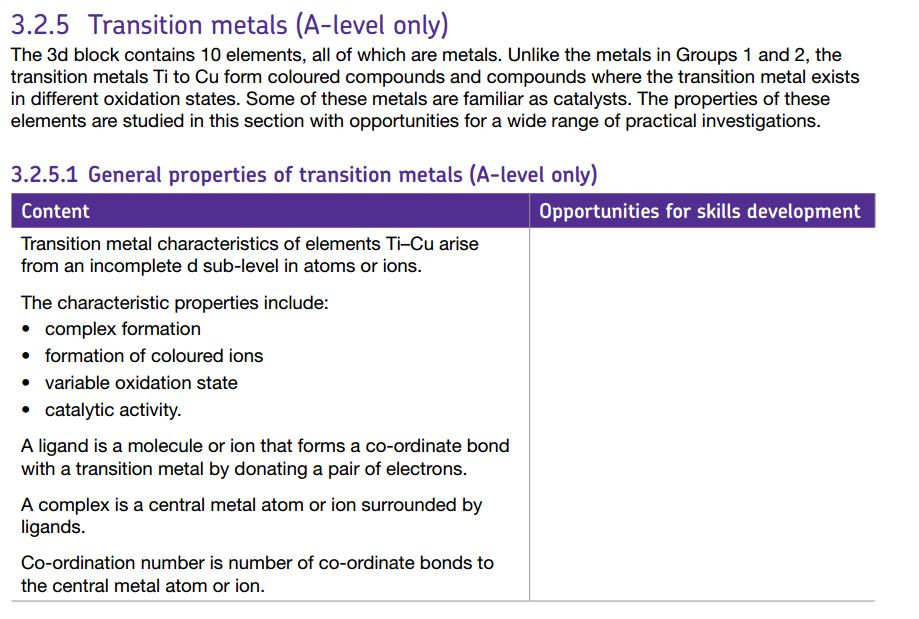
Transition metals are defined as D block elements which have at least one ion which contains an incomplete d electron sub shell.
Transition metal ions and complex ions
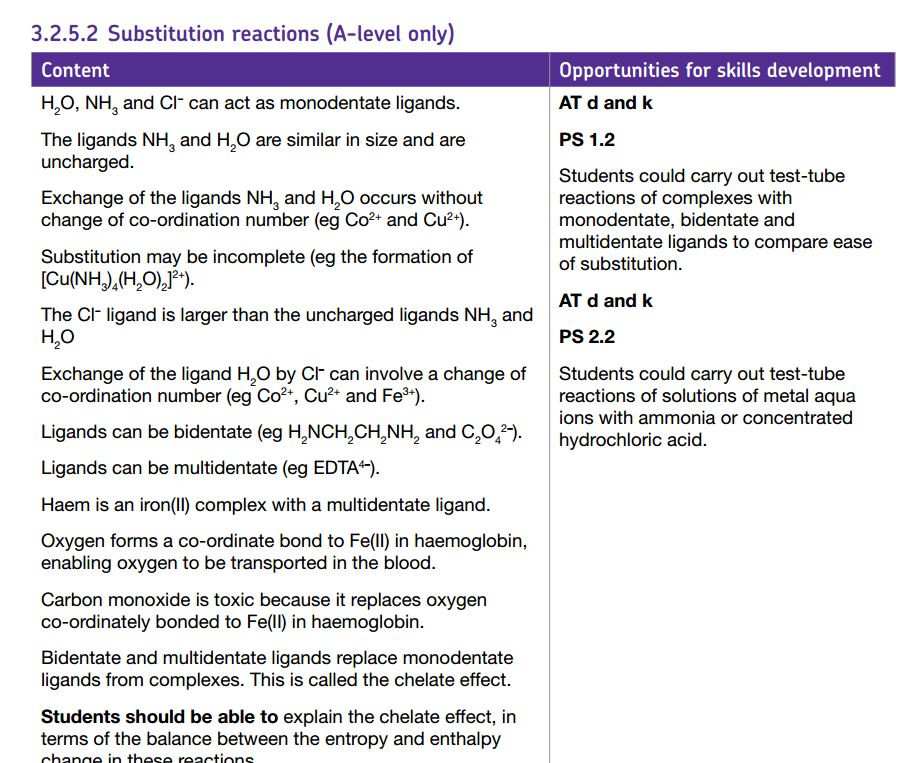
Notice how the lone pairs of electrons on the water molecules form dative covalent bonds with the central metal ion
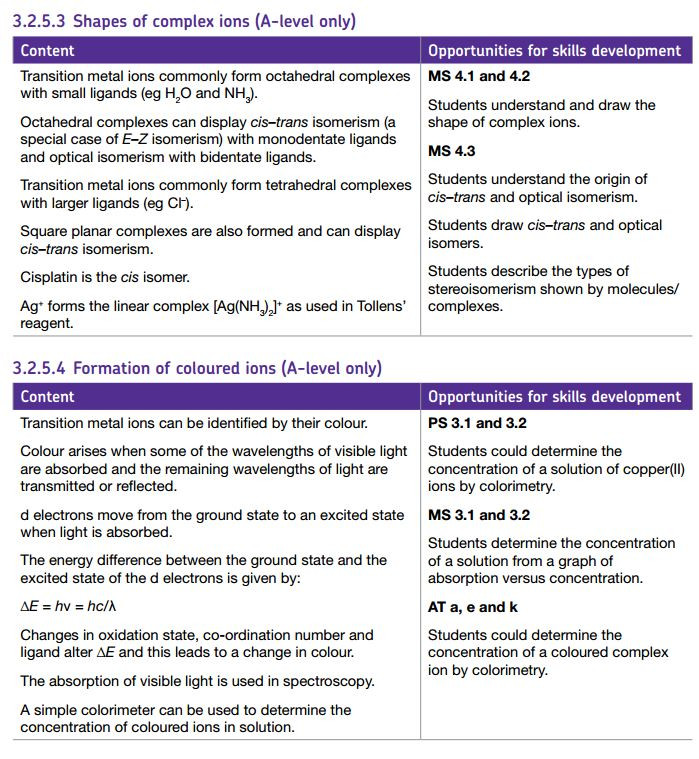
Another example of a complex ion with a coordination number of six forming a tetrahedral complex ion
in this example the ligands shown are ammonia molecule. As in the previous examples the ligands are donating a lone pair of electrons to form a dative ( coordinate) bond with the metal ion.
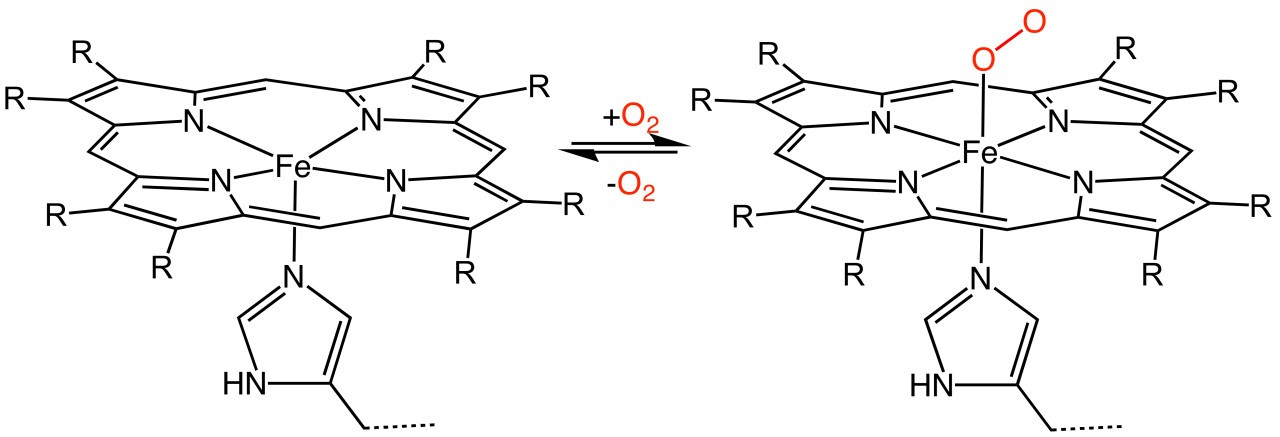
Metal ions can form complex ions - where the metal ion is surrounded by a number of other ions or molecules which bond by forming dative covalent ( or coordinate) bonds with the central metal ion.
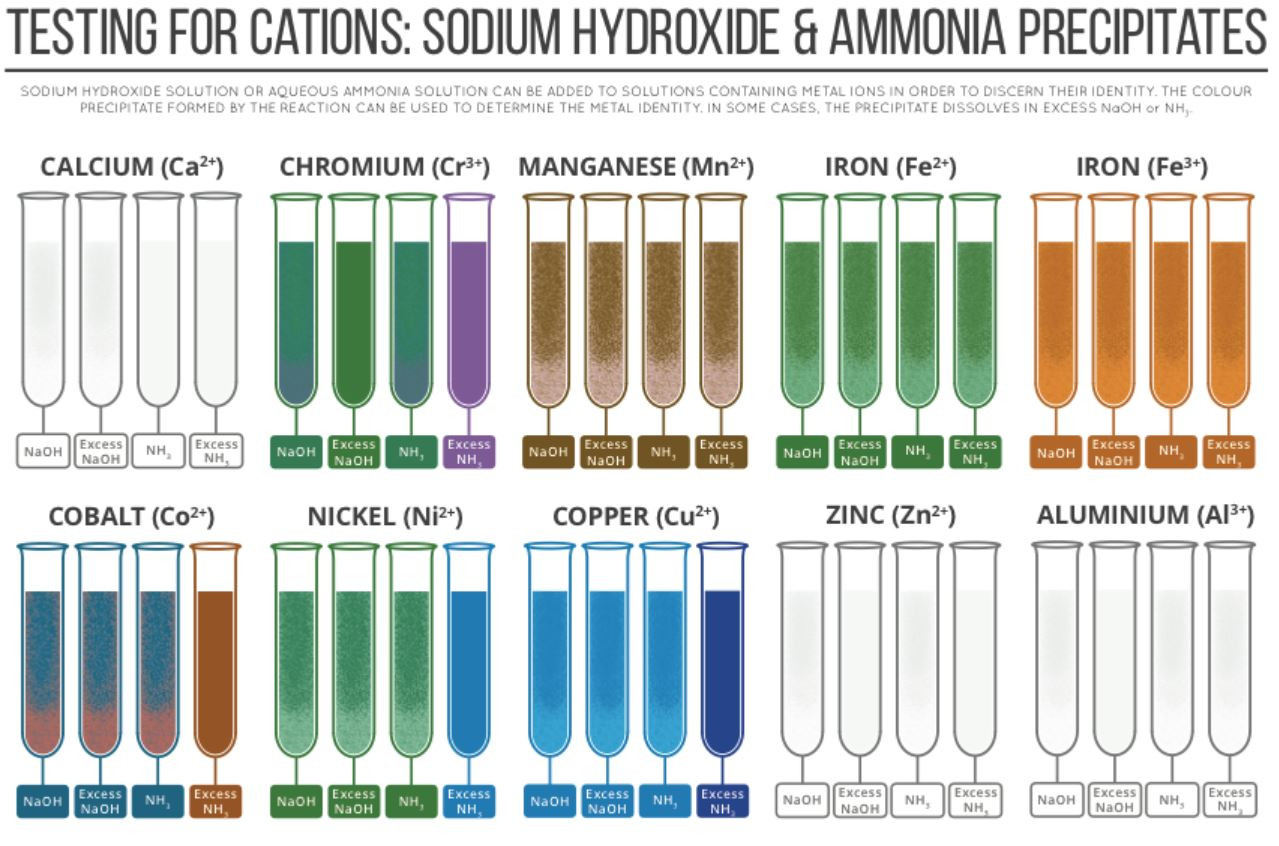
Transition metals are no exception and because (by definition) their ions have incomplete d orbitals, this leads to absorption of light in the visible spectrum and therefore gives the transition metal complex ions distinctive colours.
The colour produced by a transition metal ion will change if the ligand changes. These colour changes can be used to identify specific ions.
Why are the elements Scandium and Zinc not regarded as transition metals?
Zinc has the electron configuration [Ar] 3d104s2 . When they ionize, zinc atoms lose their 4s electrons and assume the electron configuration [Ar] 3d10 . The 3d sub shell remains full and does not meet the definition of transition element.
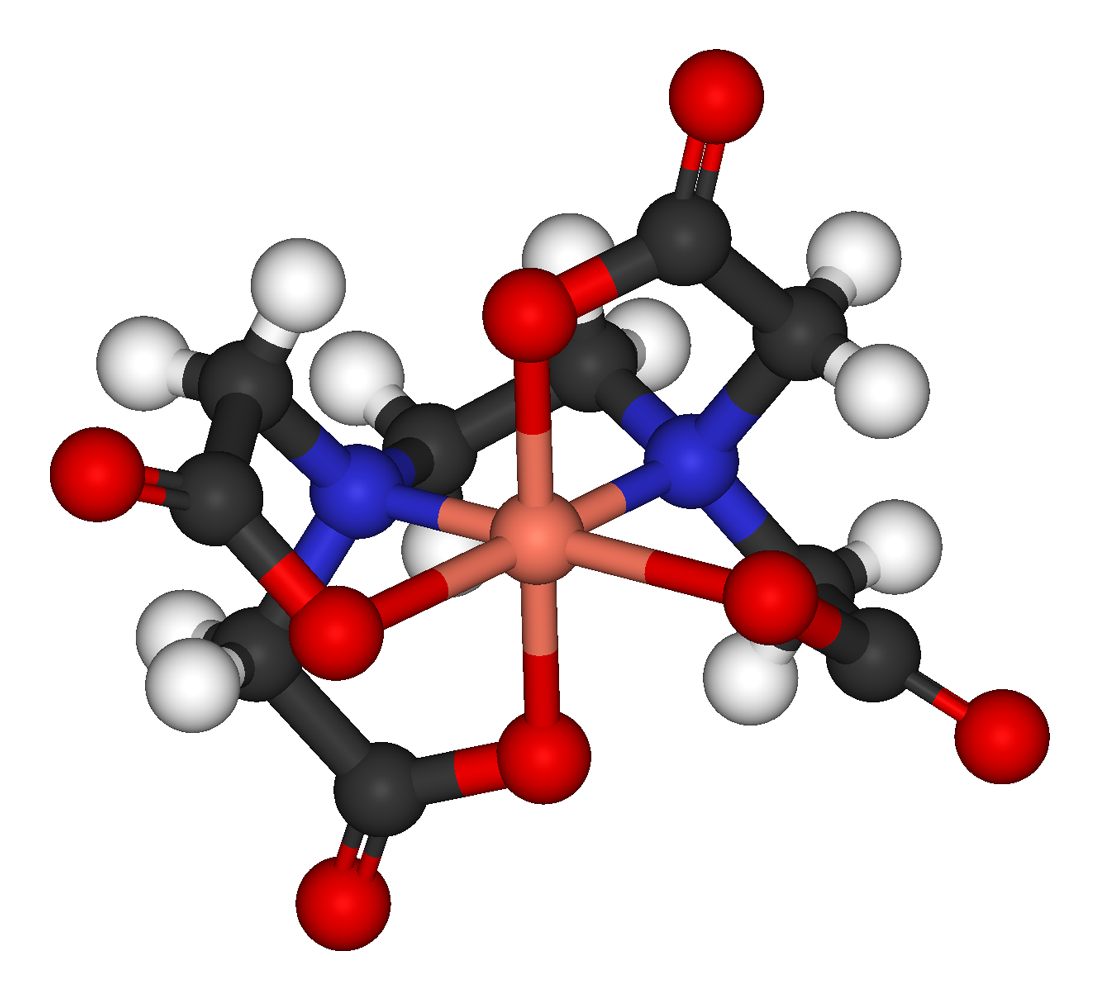
A Hexadentate ligand
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.